As per ICH Q9 “Quality Risk Management is a systematic process for the assessment, control, communication and review of risks to the quality of the product throughout its life cycle.” There are many methods and tools to perform Quality Risk Management. It is important to understand that none of the tool or set of tools are sufficient to address every situation of Quality Risk Management. Some of the important tools are as follows:
- Basic Risk Management Facilitation Methods
- Failure Mode and Effects Analysis (FMEA)
- Failure Mode, Effects and Criticality Analysis (FMECA)
- Fault Tree Analysis (FTA)
- Hazard Analysis and Critical Control Points (HACCP)
- Hazard Operability Analysis (HAZOP)
- Preliminary Hazard Analysis (PHA)
- Risk Ranking and Filtering
- Supporting Quality and Statistical Tools
Hazard and Operability Analysis (HAZOP) is a systematic and structured examination of an operation/process to evaluate and identify the problems that may lead to any undesirable consequence to the system or equipment or personnel. This is a risk assessment tool carried out during the initial project phase to have sufficient time to carry out the recommendations during the project. HAZOP can also be carried out during the Operation phase to implement modifications established during the process. Potential hazards and operation problems that may cause nonconforming products are identified using HAZOP.
Risk events are known to be caused when a deviation of the process takes place. HAZOP has been endorsed in the ICHQ9 Guideline by Quality Risk Management as one of the tools used for assessing risks in pharmaceutical sector. HAZOP can assess risks in processes, facilities and equipment. Some of the objective for carrying out the HAZOP study is to examine a design, to decide what to build, to acquire questions for the supplier, to improve safety of the organization and to check the running instructions. There are 4 phases for HAZOP execution, and these are:
- Definition Phase
- Preparation Phase
- Examination Phase
- Documentation and Follow up Phase
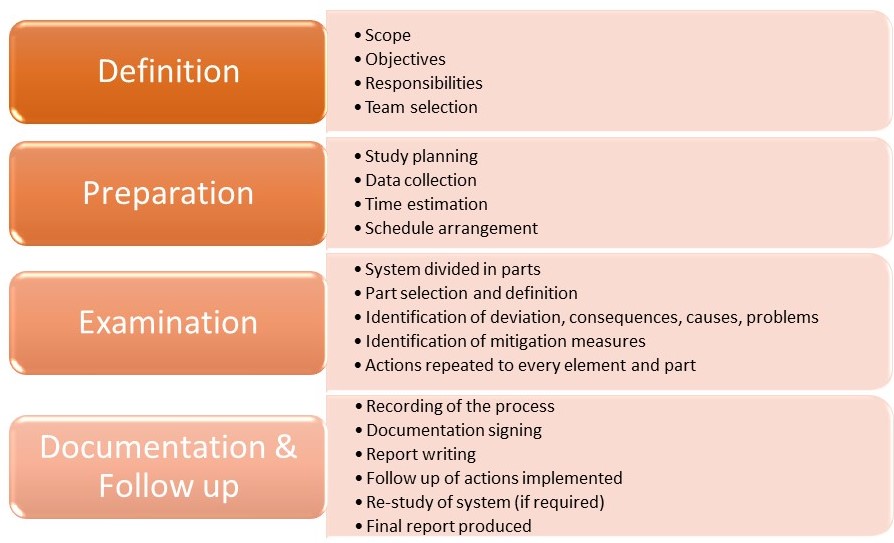
Definition Phase: Preliminary identification of the risk is carried out in the definition phase. The systems and subsystems are defined in the first step of HAZOP analysis. HAZOP team comprising of the Cross Functional Team and Subject Matter Experts (SMEs) are the team preferred for the HAZOP analysis comprising of personnel having appropriate experience and skills to carry out the process. HAZOP team is comprised of the following personnel:
- Chairman
- Scribe
- Design consultant/ Project Manager
- Production Manager
- Chemical Engineer/Chemist
- Maintenance Manager
- Electrical Engineer
- Instrument Engineer
- Quality Control Engineer
The team leader of the HAZOP team should be independent of the process/operation performance. At this phase, the team must identify the scope of the risk assessment and boundaries must be defined for the study. All the participants of the HAZOP team should contribute in the analysis.
Preparation Phase: This phase includes identifying & locating of the information & supporting data, project management preparations, identification of the users and audience of the output study and consensus of the guide words & format to be used for the study. The following information shall be available during the HAZOP study:
- Process flow diagrams
- Piping and instrumentation diagrams (P&IDs)
- Layout diagrams
- Material safety data sheets
- Provisional operating instructions
- Heat and material balances
- Equipment data sheets
- Start-up and emergency shut down procedures
HAZOP guide words are the key supporting elements for the execution of the process which stimulates imaginative thinking, helps in focusing on the study, provides elicit ideas and used for discussion purpose. Some of the guide words used are- no/not, as well as, part of, reverse, more, less, other than, early, after, before, late, etc. The guide words provide a consistent and systematic platform for brainstorming ideas for potential deviations in the process. Some of the basic HAZOP guide words used are as follows:
|
Guide word |
Meaning | Example |
|
No (not, none) |
None of the design intent is achieved | No flow when production is expected |
| More
(more of, higher) |
Quantitative increase in a parameter |
Higher temperature than designed |
|
Less (less of, lower) |
Quantitative decrease in a parameter |
Lower pressure than normal |
| As well as
(more than) |
An additional activity occurs |
Other valves closed at the same time (logic fault or human error) |
|
Part of |
Only some of the design intention is achieved |
Only part of the system is shut down |
|
Reverse |
Logical opposite of the design intention occurs |
Back flow when the system shuts down |
| Other than
(other) |
Complete substitution – another activity takes place |
Liquids in gas piping |
After defining the guide words, then the causes, safeguards and consequences are assessed which are important in mitigating the risks. Examination begins once the HAZOP guide words are selected.
Examination Phase: This phase begins when all the elements, i.e.- parts or steps of the process/system has been identified. Deviations are searched for in a systematic manner. Examination phase can be described graphically as follows:
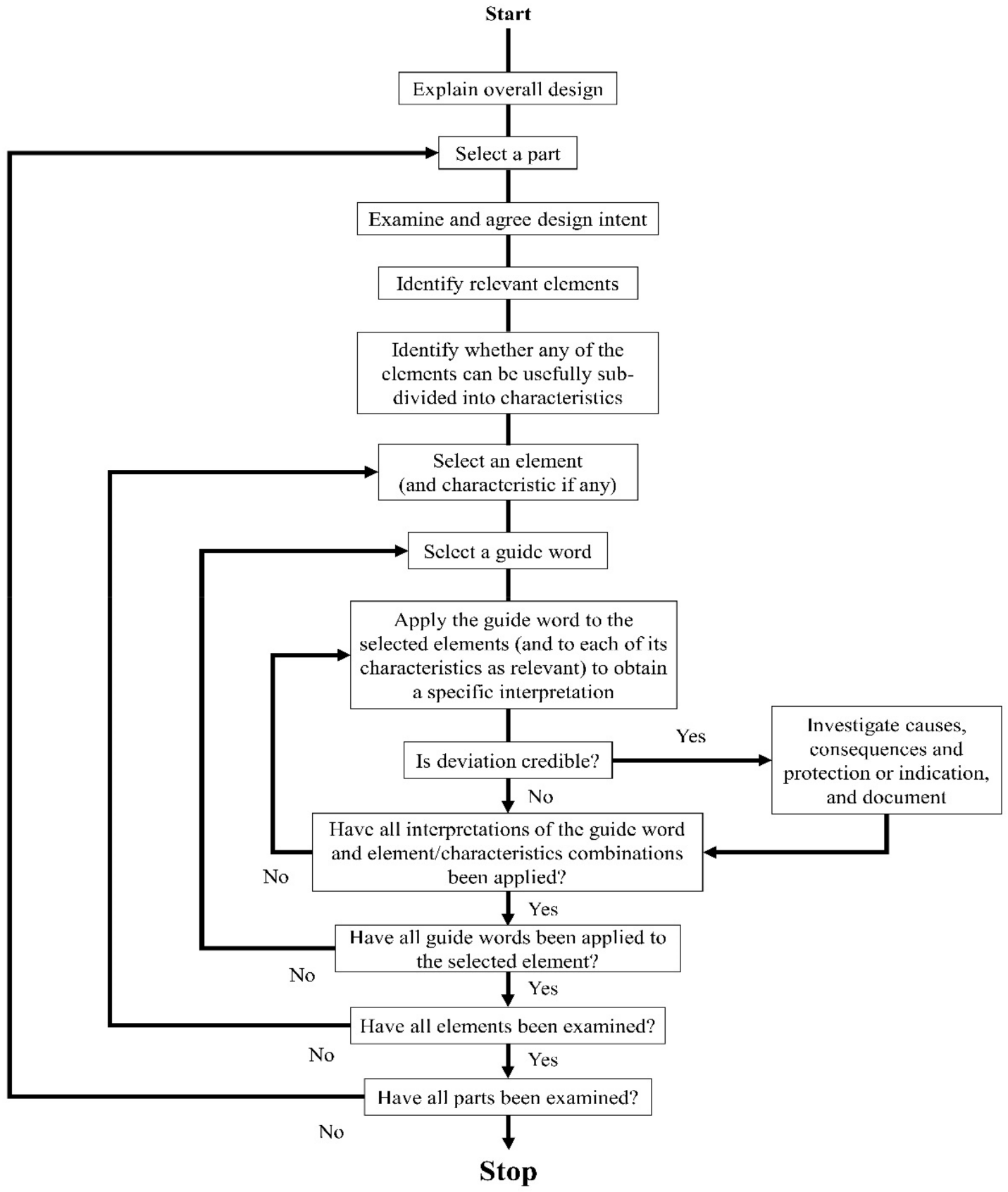
Documentation and Follow up Phase: Templates are used for recording the observations of the HAZOP analysis. The template can be modified by the team members when required to do so due to certain regulatory requirements, organizations documentation policies, traceability factor or any other factor. Creating reference files for HAZOP files are important. A delay in HAZOP analysis will delay the documentation procedure, therefore the leader must make sure that the documentation process is up to date. Following is a template used for HAZOP analysis:

No.– Unique tracking number of every entry
Guide Word– Word used to create vision of the deviation
Element– The matter (process, step, material, etc.) to which the guide word pertains
Deviation– Deviation is when the process condition departs from their original design/process intent
Guideword + Parameter = Deviation
Parameters are relevant to the conditions of the process. Some of the Parameters used related to the system are not necessary in conjunction with the guide words. Examples of process parameters are flow, pressure, mixing, temperature, stirring, level, viscosity, signal, start/stop, services, time, phase, composition, speed, particle size
Example: No + Flow = No Flow.
Possible Causes– Description on how the deviation might occur. One deviation may have several causes. It usually starts with the causes having the worst consequence
Consequences– Description on what will happen due to the deviation. This maybe due to both operation problems and process hazards
Safeguards– Preventive or reactive controls for reducing the deviations severity or likelihood. The five types of safeguards are that:
- Identifies the deviation (e.g.- detector and alarms)
- Compensates for deviations (e.g.- automatic control system to reduce vessel filling in case of overfilling)
- Prevents deviations from occurring (e.g.- Inert gas blanket in the storage area of flammable substances)
- Prevents escalation of the deviations (e.g.- trip of the activity)
- Relieves the process from hazardous deviations (e.g.- Pressure safety valves)
Comments– Key relevant rationale, data, assumptions, etc. are captured
Actions Required– Hazard mitigation or control plans (if any) are identified
Actions Assigned to– Person responsible for the action is recorded
Review meetings are necessary to monitor the actions taken. Operational feedback is required to confirm that the risk addressed are in state of control. In cases where feedback is not taken, then risk review is required to ensure that the assumptions about the risk level are still valid and also to exercise control of the new risks that may arise in the system. The output of the HAZOP analysis should be presented in a detailed way.
Types of HAZOP:
| S. No. | Type | Description |
| 1 | Process HAZOP | Technique used for assessing plants and process system |
| 2 | Human HAZOP | This is ‘family’ of specialized HAZOPs. Focus on Human errors is more than technical errors |
| 3 | Procedure HAZOP | Operational sequences and procedures are reviewed with this technique. Also called as SAFOP (Safe Operation Study) |
| 4 | Software HAZOP | Technique used for identifying all the possible errors in developing of software |
HAZOP is a very powerful analysis technique and following are some of its advantages:
- Useful in confronting the hazards which are difficult to quantify
- Is a well-defined risk analysis toll which covers almost all systems, subsystems and processes
- Human error is considered
- Multidisciplinary study
- Simple and intuitive compared to other risk management tools
- Impact of a process deviation can be assessed on the other subsystems
- Comprehensive and systematic methodology
Applications of HAZOP analysis can be found mostly in Chemical, Pharmaceutical, Oil & Gas, Nuclear and other process industries for initiating new projects/process, modification and periodic review of the operations. The effectiveness of the HAZOP study mainly depends on the experience of the leader, therefore qualified HAZOP leaders shall be facilitated for the process. To sum up, HAZOP is a qualitative technique used for identifying deviations and hazards in functioning of a system, to decide control measures for controlling the hazards and follow up the actions undertaken to ensure that they are in place. Operators shall be made aware of the hazards and operability problems for a successful HAZOP analysis. HAZOP can be applied to all sequences of operations focusing on both human errors and failures of technical systems.

.png
)