As per ICH Q9 “Quality Risk Management is a systematic process for the assessment, control, communication and review of risks to the quality of the product throughout its life cycle.” There are many methods and tools to perform Quality Risk…

This is an evident fact that for any industry to grow, it has to constantly improve its processes and quality parameters. This becomes more important in case of highly regulated and constantly advancing industries such as pharmaceutical industry. There…

In the previous article entitled Critical Process Parameters (CPP) – What’s the Buzz, we had built an understanding regarding Critical Process Parameters and how their identification and selection is based on Critical Quality Attributes (CQAs) among many other factors. Any…

Pharmaceutical manufacturing is an arduous task. With so many regulations, GMP’s, Quality Concerns, newer products, newer diseases, newer challenges coming up every now and then, it is really required that the professionals involved shall be performing the tasks with utmost…
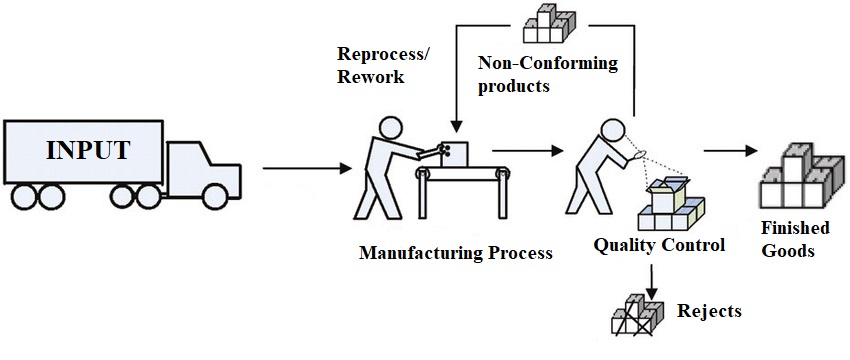
There is a popular phrase that says “Mistakes are bound to happen”, so do the non-conformances in the pharmaceutical manufacturing. Even if you have validated processes, qualified vendors, qualified equipment, calibrated instruments, in-process checks, SOPs etc. in place, possibility of…
Recent Post
- CDSCO Issues Public Notice on Disposal of Long-Pending SUGAM Applications
- India Bans High-Dose Nimesulide: What You Need to Know
- Compounding of Offences under the Drugs and Cosmetics Act, 1940: What Stakeholders Need to Know
- 🚨 CDSCO Directive on Immediate Inspections as per Revised Schedule M – Is Your Facility Ready?
- CDSCO Issues Regulatory Clarification on Combi-Pack Approvals: Key Insights for Injectable Manufacturers

.png
)