Manual Visual Inspection is an important step in pharmaceutical manufacturing which aids in the detection of various formulation defects. The defects are observed in the all types of formulations viz. tablets, capsules, injectables etc. To effectively observe the defects there…


Manual Visual Inspection is an important step in pharmaceutical manufacturing which aids in the detection of various formulation defects. The defects are observed in the all types of formulations viz. tablets, capsules, injectables etc. To effectively observe the defects there…
The management and disposal of the waste produced in a pharmaceutical manufacturing firm has always been a matter of concern. The waste includes but not limited to in-process wastes, expired products, returned material, manufacturing spillages etc. There has been a…

The objective of any pharmaceutical manufacturing firm is to manufacture a product which fulfils all the requirements of being a product of quality and is also safe and efficacious. The quality of material shall be maintained not only during the…
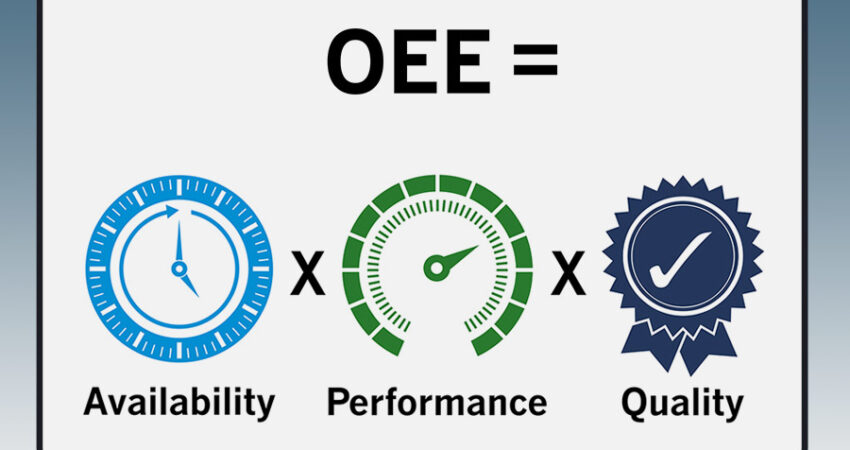
Lean manufacturing makes use of various lean tools for improvement in production effectiveness and efficiency. The main goal of such an approach is to get the maximum output by making use of less time, less effort and fewer resources, that…
Recent Post
- CDSCO Issues Public Notice on Disposal of Long-Pending SUGAM Applications
- India Bans High-Dose Nimesulide: What You Need to Know
- Compounding of Offences under the Drugs and Cosmetics Act, 1940: What Stakeholders Need to Know
- 🚨 CDSCO Directive on Immediate Inspections as per Revised Schedule M – Is Your Facility Ready?
- CDSCO Issues Regulatory Clarification on Combi-Pack Approvals: Key Insights for Injectable Manufacturers

.png
)