In the previous article entitled Critical Process Parameters (CPP) – What’s the Buzz, we had built an understanding regarding Critical Process Parameters and how their identification and selection is based on Critical Quality Attributes (CQAs) among many other factors. Any…


In our previous edition to this article entitled “Hand Sanitizer and COVID – 19 – Understanding the Misunderstood”, we had informed you how washing hands with plain soap and water is considered as more effective than hand sanitizers to prevent…

Pharmaceutical manufacturing is an arduous task. With so many regulations, GMP’s, Quality Concerns, newer products, newer diseases, newer challenges coming up every now and then, it is really required that the professionals involved shall be performing the tasks with utmost…

Apr 9, 2020 The Central Drugs Standard Control Organization, Government of India, has requested all State and Union Territory Drug Controllers to expedite the licensure procedure to manufacture oxygen for medical use. This is considered as a welcome move…
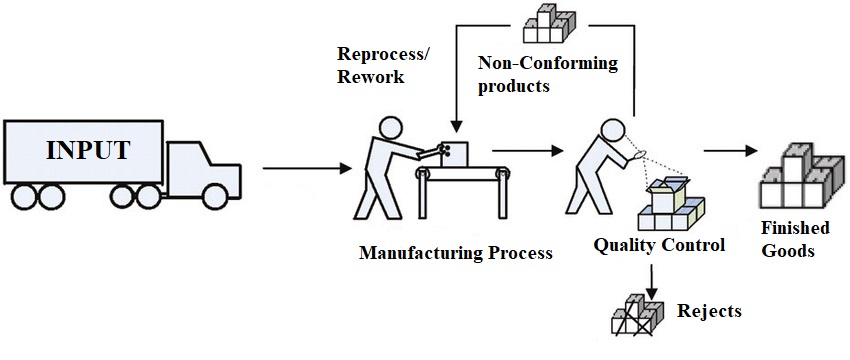
There is a popular phrase that says “Mistakes are bound to happen”, so do the non-conformances in the pharmaceutical manufacturing. Even if you have validated processes, qualified vendors, qualified equipment, calibrated instruments, in-process checks, SOPs etc. in place, possibility of…
Recent Post
- CDSCO Issues Public Notice on Disposal of Long-Pending SUGAM Applications
- India Bans High-Dose Nimesulide: What You Need to Know
- Compounding of Offences under the Drugs and Cosmetics Act, 1940: What Stakeholders Need to Know
- 🚨 CDSCO Directive on Immediate Inspections as per Revised Schedule M – Is Your Facility Ready?
- CDSCO Issues Regulatory Clarification on Combi-Pack Approvals: Key Insights for Injectable Manufacturers

.png
)