We help in registration and licensure of medical devices and maintaining the state of compliance with the newer regulations
India has published Medical Devices Rules, 2017. These meticulously prepared have been drafted with the intention to distinguish medical devices from pharmaceuticals for the purpose of regulation. As per the new rules the medical devices have been categorized into following classes on the basis of risk assessment:
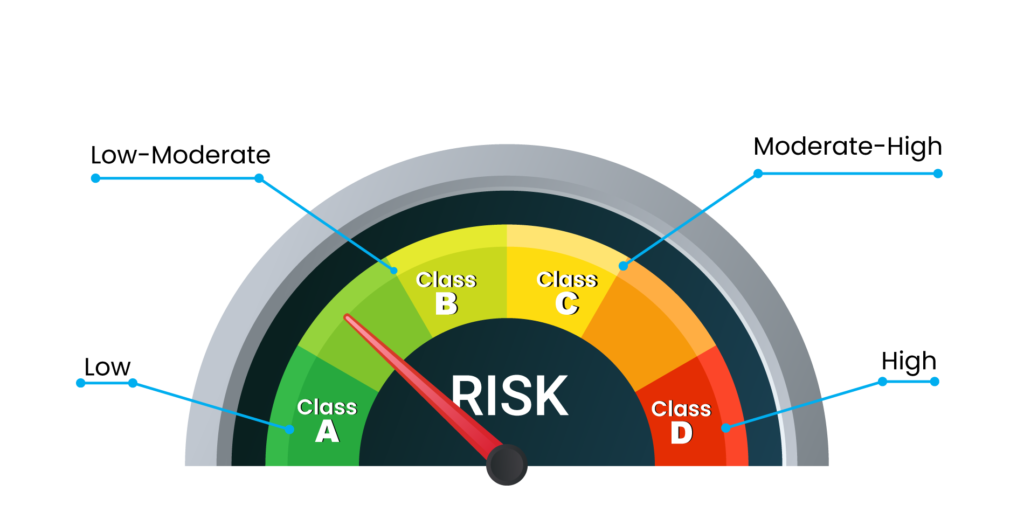
Medical devices have been classified into A, B, C and D categories where the risk factor involved increases from A to D.
Class A – Low
Class B – Low-Moderate
Class C – Moderate-High
Class D – High
Low-risk devices include equipment like thermometers whereas high-risk devices include pacemakers, heart valves and others. The devices are further classified as surgical or non-surgical devices based upon their invasiveness. License for class A devices is easy to obtain as compared to class D devices.
The application for license to import Class A or Class B medical devices from Unregulated Jurisdictions (defined below) can be granted on the strength of a free sale certificate and either of published safety and performance data or clinical investigation in the country of origin. However, an application for import of Class C or Class D medical devices from Unregulated Jurisdictions can be granted only after its safety and effectiveness has been established through clinical investigation in India.
Unregulated Jurisdictions are jurisdictions other than Australia, Canada, Japan, European Union Countries, or the United States of America.
Similarly, for applications for grant of license to manufacture – Class A medical devices do not require prior audit by third party or official inspection; Class B medical devices require prior audit by third party but do not require official inspection, and; Class C or Class D medical devices require prior official inspection.
The application for manufacture of Class A or Class B medical device will be assessed by the State licensing authority whereas the application for manufacture of Class C or Class D medical device will be assessed by DCGI.
Our Services
For Manufacturers:
Services offered to nationwide manufacturers are as follows:
- Registration of Medical Devices
- Application filing including documents preparation for Marketing Authorization
- Manufacturing Licenses
- Test License
For importers:
Services offered to overseas manufacturers and importers are as follows:- Authorized Agent/Registration Holder Support
- Marketing Authorization
- Clinical trials
- Registration Certificate
- Import License
- Re-Registration
- Variations/ Post Approvals/ Amendments
For Sale :
Service offered to retailers and wholesellers are as follows us
- Registration certificate on MD-42 for sale of medical devices in India

.png
)