We support our clients in implementing Quality Management Systems (QMS) and train the staff in further strengthening of the QMS.
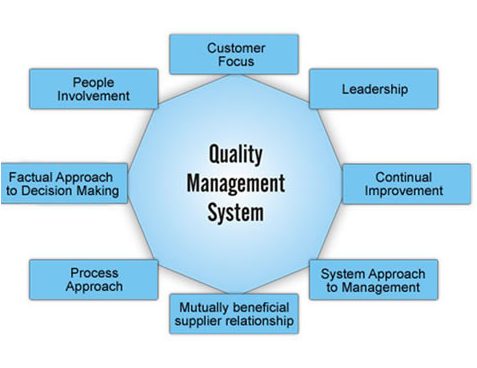
Pharmaceutical Quality Management System is the foundation for enabling the organization to operate in a compliant manner. A Pharmaceutical Quality Management System (QMS) develops and ensures quality procedures in various product life cycle stages such as manufacturing and product testing. It can be defined as a set of policies, processes, and procedures required for planning and execution (production/development/service) in the core process areas of an organization. A QMS integrates the various internal processes within the organization and provides a process approach for project execution. The QMS enables organizations to identify, measure, control, and improve the various core processes, which ultimately leads to improved performance.
It includes all the critical stages of drug manufacturing, including:
- Formulation
- Method development
- Facilities
- Utility system
- Equipment
It ensures that the final product is according to customer requirements, as well as regulatory requirements to which the manufacturer is obliged to follow. It uses monitoring methods such as Quality Assurance to prevent quality deviation and emphasize documentation to record all problems and their solutions.
The Quality Management System (QMS) is not a standard of its own. Its definition, applicability, and practical implementation rely on other approved standards. It’s the organization’s responsibility to adopt the relevant standards, which depend on many factors, including geographical location, product type, and target market.
Some of the most common standards and regulations applicable to the Pharmaceutical Quality Management System (QMS) are described below:
- International Organization for Standardization (ISO)
- Current Good Manufacturing Practice (cGMP)
- ICH Guideline Q10 on Pharmaceutical Quality System
Services offered:
- We implement all the facets of Quality management Systems as per the standards agreed.
- We train and support the staff of our clients in implementation of the QMS at their respective sites.
- We firmly believe that a third person can only train and guide but the baton has to be in the hands of the regular staff carrying out the daily activities.
- This not only helps the firm to turn the rookies into the assets but also make them more confident while facing the audits.

.png
)