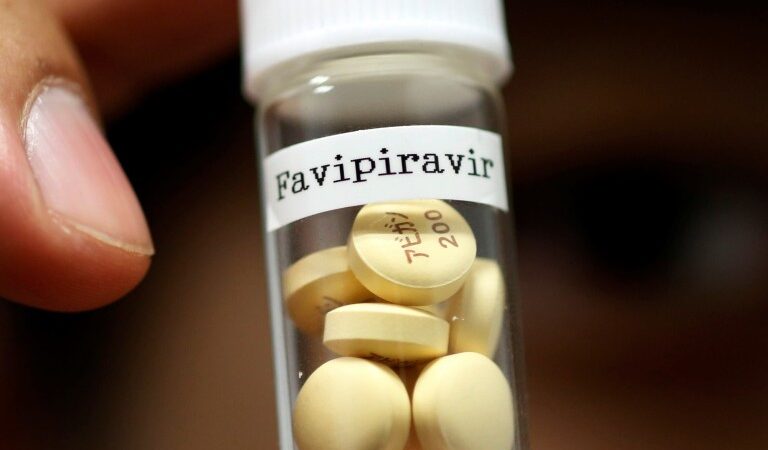
April 30, 2020 Glenmark Pharmaceuticals announced that it has received the Drugs Controller General (I) permission to conduct clinical trial on Favipiravir. As on date, Glenmark is the first pharmaceutical company in India to be given an approval by…

April 28, 2020 The International Coalition of Medicines Regulatory Authorities (ICMRA) on Tuesday has stated “We have stepped up our global collaboration to facilitate and expedite the development and evaluation of diagnostics and therapeutics, including possible vaccines, against SARS-CoV2”. ICMRA…

Since the arrival of Coronavirus disease, there has been a flood of advices, do's and dont's reaching everyone of us. Social media is full of home remedies, talismans and what not to treat and prevent coronavirus disease. By this article…

Since the advent of Coronavirus disease, the whole world has gone crazy after two things i.e Hand Sanitizers and Face Masks. The fear of the disease has gripped everyone so much that people who didn’t have any exposure to the…

April 18, 2020 Central Drugs Standard Control Organization (CDSCO), the Government of India Drug Regulatory Agency has permitted the conduct of clinical trial of Convalescent Plasma In COVID-19 Patients. The trial protocol was developed and submitted by Indian Council of…
Recent Post
- CDSCO Issues Public Notice on Disposal of Long-Pending SUGAM Applications
- India Bans High-Dose Nimesulide: What You Need to Know
- Compounding of Offences under the Drugs and Cosmetics Act, 1940: What Stakeholders Need to Know
- 🚨 CDSCO Directive on Immediate Inspections as per Revised Schedule M – Is Your Facility Ready?
- CDSCO Issues Regulatory Clarification on Combi-Pack Approvals: Key Insights for Injectable Manufacturers

.png
)