When a new drug enters the market, it brings with it both hope and uncertainty. Clinical trials, despite their scientific rigor, are limited in scope — they can’t predict how a drug will behave in real-world conditions, across varied patient…

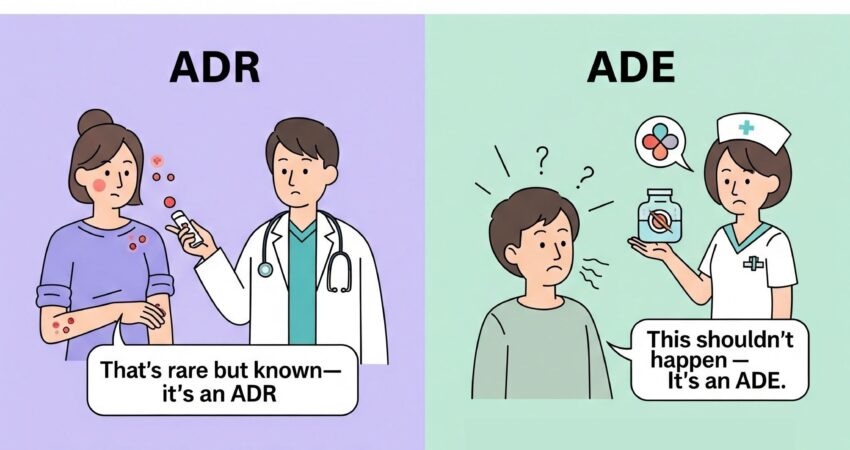
In the pharmaceutical world, Adverse Drug Reactions (ADRs) are not just unfortunate events — they’re regulatory red flags, patient safety threats, and potential business disasters. Why is this so critical? Because a single overlooked ADR can lead to product…

Regulations in the pharmaceutical sector are dynamic, evolving to keep pace with new products and technologies. The Central Drugs Standard Control Organization (CDSCO), Government of India, continually updates its rules to ensure drug quality, safety, and efficacy. A significant development…
Recent Post
- CDSCO Issues Public Notice on Disposal of Long-Pending SUGAM Applications
- India Bans High-Dose Nimesulide: What You Need to Know
- Compounding of Offences under the Drugs and Cosmetics Act, 1940: What Stakeholders Need to Know
- 🚨 CDSCO Directive on Immediate Inspections as per Revised Schedule M – Is Your Facility Ready?
- CDSCO Issues Regulatory Clarification on Combi-Pack Approvals: Key Insights for Injectable Manufacturers

.png
)