April 30, 2020
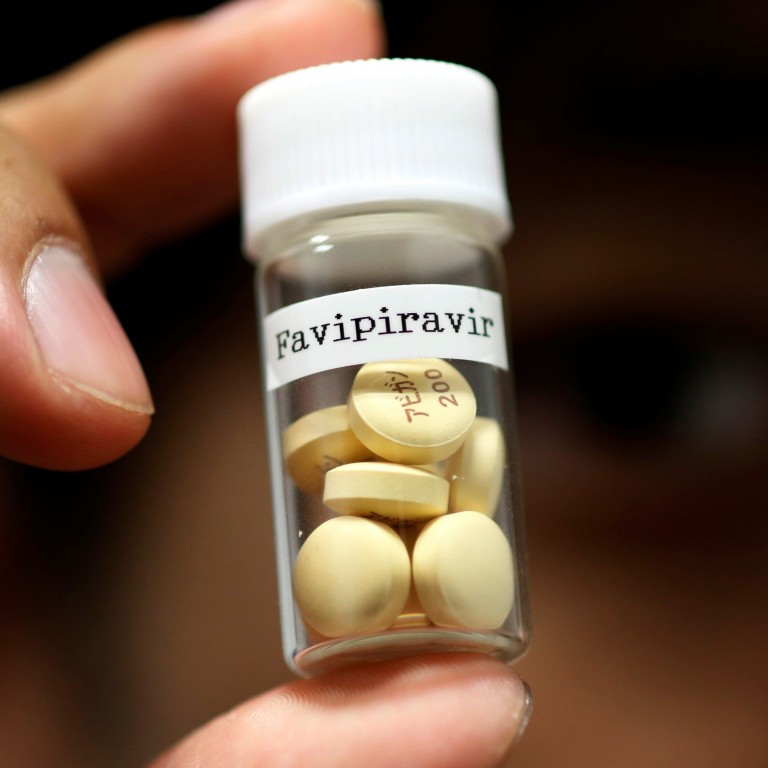
Glenmark Pharmaceuticals announced that it has received the Drugs Controller General (I) permission to conduct clinical trial on Favipiravir. As on date, Glenmark is the first pharmaceutical company in India to be given an approval by the regulator to start the trial on COVID-19 patients in India.
Favipiravir is a generic version of Avigan of Fujifilm Toyama Chemical, Japan, a subsidiary of Fujifilm Corporation. Favipiravir is already being used in the treatment of Covid-19 in few countries. Fujifilm too has started phase 3 trial on this drug and also increased its production.
Having internally developed the API and the formulations for the product, Glenmark filed the product for clinical trials with the DCGI and has received approval for conducting the trial on mild to moderate patients. As per the clinical trial protocol approved, 150 subjects with mild to moderate COVID-19 will be randomized in the study in a 1:1 ratio to Favipiravir with standard supportive care or standalone standard supportive care. Treatment duration is a maximum of 14 days and the total study duration will be maximum for 28 days from randomization.

.png
)