Test License for import of drugs in India is the license to import drugs to conduct clinical trial or bioavailability or bioequivalence study or for examination, test and analysis. The only condition is that the drugs imported on test license cannot be used for commercial purpose.
The present article is in continuation to our previous article where in we explained the procedure for obtaining test license for manufacture of new drug in India
For in-depth understanding we need to understand following concepts:
What is a new drug: To understand the definition of new drug as per New Drug and Clinical Trial Rules, 2019, a new drug may be defined as:
- a drug, including active pharmaceutical ingredient or phytopharmaceutical drug, which has not been used in the country to any significant extent, except in accordance with the provisions of the Act and the rules made thereunder, as per conditions specified in the labelling thereof and has not been approved as safe and efficacious by the Central Licencing Authority with respect to its claims; (Simply stating – an IND or a drug approved in other countries but not approved in India) or
- a drug approved by the Central Licencing Authority for certain claims and proposed to be marketed with modified or new claims including indication, route of administration, dosage and dosage form; (Subsequent New Drug) or
- a fixed dose combination of two or more drugs, approved separately for certain claims and proposed to be combined for the first time in a fixed ratio, or where the ratio of ingredients in an approved combination is proposed to be changed with certain claims including indication, route of administration, dosage and dosage form (FDC); or
- a modified or sustained release form of a drug or novel drug delivery system of any drug approved by the Central Licencing Authority; or
- a vaccine, recombinant Deoxyribonucleic Acid (r-DNA) derived product, living modified organism, monoclonal anti-body, stem cell derived product, gene therapeutic product or xenografts, intended to be used as drug;
Explanation: The drugs, other than drugs referred to in sub-clauses (iv) and (v), shall continue to be new drugs for a period of four years from the date of their permission granted by the Central Licencing Authority and the drugs referred to in sub-clauses (iv) and (v) shall always be deemed to be new drugs;
What is an old drug: Any drugs which do not fall in the definition of new drug and has already been approved by CDSCO for marketing in India for more than four years is defined as an Old drug.
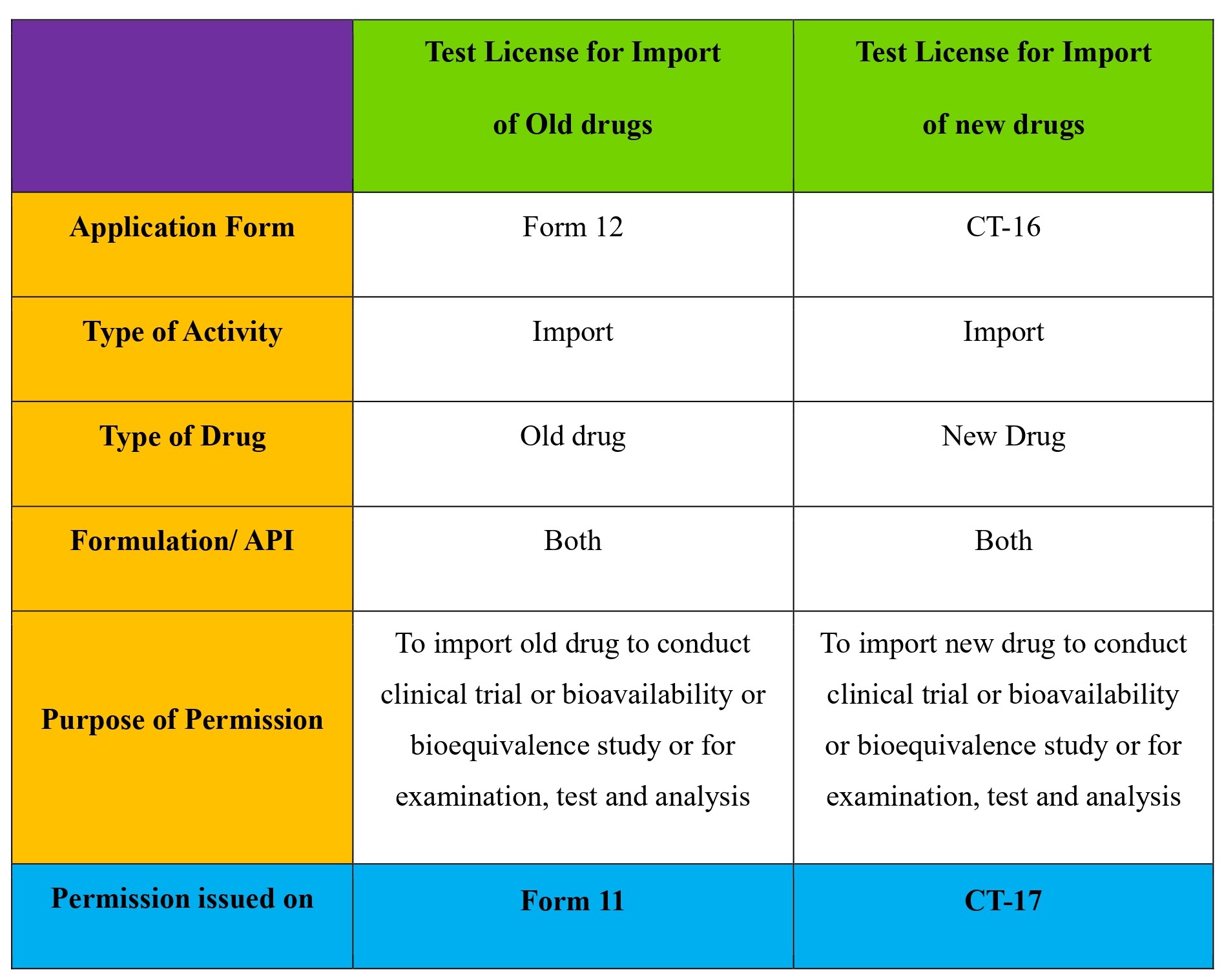
What is a Test license for import of new drug: Test license for new drug is the license granted by Central Drugs Standards Control Organization (CDSCO) on CT-17 to import a new drug for the purpose of carrying out clinical trial or bioavailability or bioequivalence study or for examination, test and analysis.
What is a Test license for import of old drug: Test license for old drug is the license granted by Central Drugs Standards Control Organization (CDSCO) on Form 11 to import an old drug for the purpose of carrying out clinical trial or bioavailability or bioequivalence study or for examination, test and analysis.
Is there a difference between test license application for importing old drug and new drug: Yes, there is a difference as for old drug you need to make an application on Form 12 and you will be issued Form 11 by CDSCO. However, for new drugs the application is to be made on CT-16 and CT-17 will be issued by CDSCO.
Where to apply for CT-17 or Form 11: Applications for CT-17 or Form 11 are made on www.nsws.gov.in after registration on this portal. For any support in filing the application you may contact us on vaayath@gmail.com
What is the fee for the application: Application fee for CT-17 or Form 11 is Rs 5000/- per application per strength. Eg. For a product applied with one strength, the fee is Rs 5000/-, for a product with 4 strengths, the fee will be Rs 20000/-.
What is the validity of a Test license: A test license is valid for a period of three years from the date of issuance.
Which documents are required for Form 11 and CT-17 applications: Documents required for Form – 11 and CT-17 are as follows:

What is the normal processing time for applications of test licenses for import: Normally it is done within 15 days.
Can we use the drugs imported on test license for commercial purpose: The products imported on test license are only for the purposes of conducting clinical trial or bioavailability and bioequivalence study or for examination, test and analysis and no part of it shall be sold in the market or supplied to any other person or agency or institution or organisation.
What is to be done with left over quantities of the drugs imported on test license after finishing of the study: The left-over quantities shall be destroyed and proper records of destruction shall be maintained.
We hope with the above information most of your concerns w.r.t. test licensing procedure for import of old and new drugs is cleared. In case any concern still persists, you can write to us on vaayath@gmail.com. We will be happy to respond.
For any support or guidance in filing of the test license applications or new drug approvals please feel free to contact.

.png
)
