In the pharmaceutical world, Adverse Drug Reactions (ADRs) are not just unfortunate events — they’re regulatory red flags, patient safety threats, and potential business disasters.
Why is this so critical?
- Because a single overlooked ADR can lead to product recalls, regulatory penalties, and long-term brand damage.
So, Whether you’re a startup or a large-scale manufacturer, you need to understand, detect, and manage ADRs and ADEs proactively.
📚 Many people confuse ADRs with Adverse Drug Events (ADEs). Let’s clear that up!
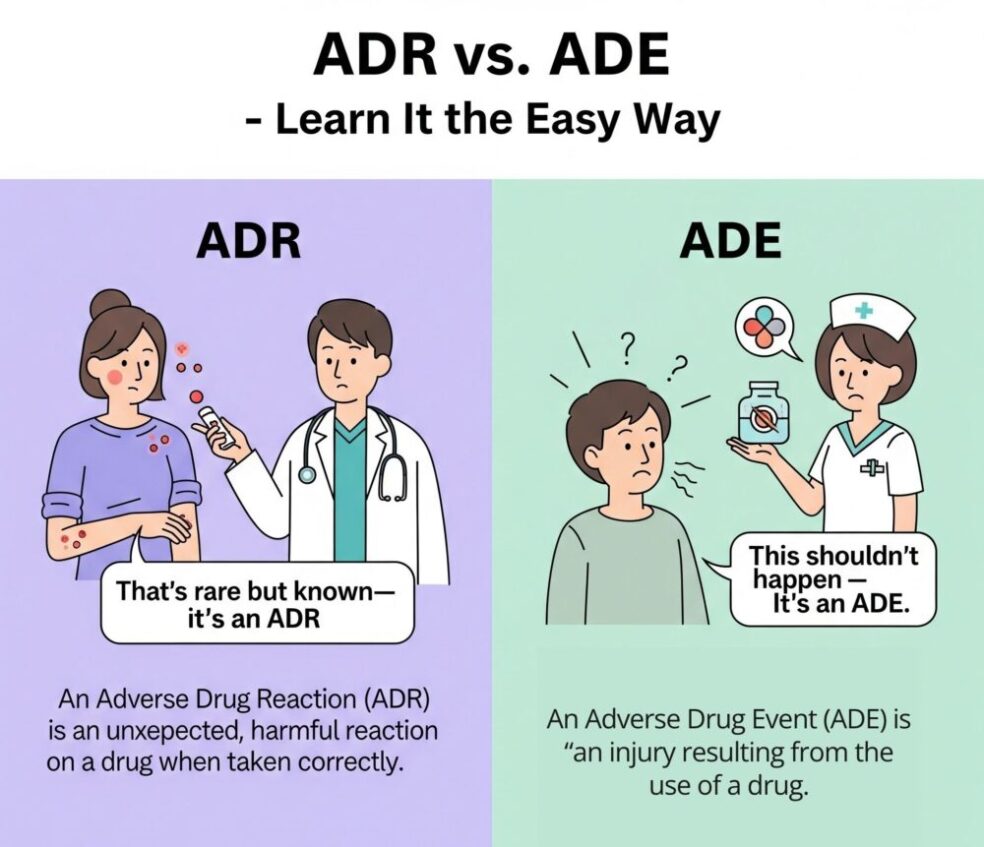
🤔ADR vs. ADE – What’s the Difference?
| 🔸 | ADR (Adverse Drug Reaction) | ADE (Adverse Drug Event) |
| 📖 Definition | Noxious, unintended response to a medicine occur at normal dose. | Any injury from drug use – includes overdoses, errors |
| 🔎 Predictability | Often unpredictable | Sometimes preventable |
| 🕒 Timing | After correct administration | At any point – prescribing, dispensing, administration |
| ⚠️ Examples | Rash from amoxicillin; nausea from metformin | Overdose, wrong drug, missed dose, wrong route |
🔔 Important: Every ADR is an ADE, but not every ADE is an ADR.
Examples:
- Case 1: A paracetamol overdose leading to liver failure = ADE, not ADR
- Case 2: A patient develops rash after standard dose of cefixime = ADR
- Case 3: A nurse administers insulin to the wrong patient = Preventable ADE
👩⚕️ Why ADR/ADE Reporting Matters?
Ignoring ADRs is no longer an option. In 2023, Ministry of Health and Family Welfare, Government of India has mandated that every pharma manufacturer shall be having working Pharmacovigilance system in place through clause no. 6.11. of Revised Schedule M. Further, in 2025, Government of India published the guidance document providing clear guidance on the implementation of PV system. Hence, pharma companies are legally and ethically bound to report serious and unexpected reactions.
Failing to manage ADRs properly can lead to:
- Product suspension or recall
- Loss of manufacturing license
- Legal liabilities
- Damaged patient trust
Types of ADRs Every Pharma Company Should Know
✅ 1. Type A – Associated with Low mortality and High Morbidity
- Most common (up to 70% of all ADRs)
- Predictable, related to the drug’s pharmacological action
- Dose-dependent – often preventable with correct dosing
Examples:
- Hypoglycemia due to insulin
- Drowsiness from antihistamines
⚠️ 2. Type B – Associated with High mortality and Low Morbidity
- Unpredictable and not dose-related
- Often related to hypersensitivity or immune response
- Rare but serious
Examples:
- Anaphylaxis from penicillin
- Stevens-Johnson syndrome from antiepileptics
🔁 3. Type C (Chronic)
- Reactions that occur after prolonged use
- Can be dose- and time-related
Examples:
- Adrenal suppression due to long-term corticosteroid use
- Tardive dyskinesia from prolonged antipsychotic use
⏳ 4. Type D (Delayed)
- Occur sometime after drug use
- Difficult to trace immediately
Examples:
- Carcinogenesis
- Teratogenesis (birth defects)
❌ 5. Type E (End of Use / Withdrawal Reactions)
- Happens when the drug is suddenly stopped
Examples:
- Opioid withdrawal symptoms
- Rebound hypertension after stopping clonidine
🤔 6. Type F (Failure of Therapy)
- The drug doesn’t work as expected
Examples:
- Antimalarial failure due to resistance
- Ineffective antibiotics due to drug interactions
🛠️ How Vaayath Helps Companies Stay ADR-Compliant?
At Vaayath Consulting Services, we offer full-spectrum Pharmacovigilance Solutions.
- Setup of ADR reporting systems.
- Preparation & submission of ICSRs, PSURs etc.
- Signal detection and trend analysis.
- SOP development, PV audits, and QPPV support.
- Training for your internal team to handle ADRs correctly.
- Risk management to regulatory submissions Support.
For more Information, Contact us at info@vaayath.com

.png
)